Table of Contents
What is heat? 🌡️
Humans have been aware of the sensation of “heat” or “cold” since the dawn of time: evolution has equipped almost all organisms with a built-in basic perception of the temperature level so to speak. Like all our sensations which emerged even before the development of intelligence, this too is geared towards our native natural environment and its opportunities and dangers. Immediately and intuitively wanting to recognise from this what the nature of the processes associated with “heat” is can lead to deception, as with all our senses. In order to better arrange the interrelationships, each early civilisation developed its own abstract concepts relating to “heat”. While this enabled useful technology in some places, these were largely based on mythical accounts which were often far more misleading (an example is the "Phlogiston" theory of heat, which was very popular until the 18th century).
In the course of the 19th century, a clear picture of the “nature/essence of heat” gradually emerged, to which James Prescott Joule contributed a key insight: He recognised that “heat” was nothing other than a form of energy and can also be generated from other forms of energy - and conversely transformed into these. From this basic insight it did not take long to reach an even deeper understanding of the character of the energy form “heat”, which James Clerk Maxwell ultimately formulated in a brilliant way and which is still generally accepted today.
To explain further, here we will adopt an unusual approach: because Maxwell's statements on this matter are so brilliantly clear, precise and yet understandable for everyone, we have reproduced these here (with some modern improvements). (http:\\books.google.com)
From: Maxwell, Theory of Heat.
I Chapter: 1 Introduction
The distinction between hot bodies and cold ones is familiar to all… The words hot, warm, cool, cold, are associated in our minds with a series of sensations which we suppose to indicate a corresponding series of states of an object with respect to heat.
We use these words, therefore, as the names of these states of the object, or, in scientific language, they are the names of Temperatures, the word hot indicating a high temperature, cold a low temperature, and the intermediate terms intermediate temperatures…
Since the state of a body may vary continuously from cold to hot, we must admit the existence of an indefinite number of intermediate states, which we call intermediate temperatures…
The temperature of a body, therefore, is a quantity which indicates how hot or how cold the body is.
When we say that the temperature of one body is higher or lower than that of another, we mean that the first body is hotter or colder than the second, but we also imply that we refer the state of both bodies to a certain scale of temperatures…When we say that the temperature of one body is higher or lower than that of another, we mean that the first body is hotter or colder than the second, but we also imply that we refer the state of both bodies to a certain scale of temperatures… By the use, therefore, of the word temperature, we fix in our minds the conviction that it is possible, not only to feel, but to measure, how hot a body is. Words of this kind … are called scientific terms.
… <because of the subjective influences on our sensations > that for all scientific purposes we prefer to form our estimate of the state of bodies from their observed action on some apparatus whose conditions are more simple and less variable than those of our own senses.
The properties of most substances vary when their temperature varies. Some of these variations are abrupt, and serve 1) to indicate particular temperatures as points of reference; others are continuous, and serve to measure other temperatures by comparison with the temperatures of reference.
For instance, the temperature at which ice melts is found to be always the same under ordinary circumstances, though, as we shall see, it is slightly altered by change of pressure. The temperature of steam which issues from boiling water is also constant when the pressure is constant2).
These two phenomena therefore — the melting of ice and the boiling of water — indicate in a visible manner two temperatures which we may use as points of reference, the position of which depends on the properties of water and not on the conditions of our senses3).
Other changes of state which take place at temperatures more or less definite, such as the melting of wax or of lead, and the boiling of liquids of definite composition, are occasionally used to indicate when these temperatures are attained, but the melting of ice and the boiling of pure water at a standard pressure remain the most important temperatures of reference in modern science.
These phenomena of change of state serve to indicate only a certain number of particular temperatures. In order to measure temperatures in general, we must avail ourselves of some property of a substance which alters continuously with the temperature. The volume of most substances increases continuously as the temperature rises, the pressure remaining constant There are exceptions to this rule, and the dilatations of different substances are not in general in the same proportion ; but any substance in which an increase of temperature, however small, produces an increase of volume may be used to indicate changes of temperature.
For instance, mercury and glass both expand when heated, but the dilatation of mercury is greater than that of glass. Hence if a cold glass vessel be filled with cold mercury, and if the vessel and the mercury in it are then equally heated, the glass vessel will expand, but the mercury will expand more, so that the vessel will no longer contain the mercury. If the vessel be provided with a long neck, the mercury forced out of the vessel will rise in the neck, and if the neck is a narrow tube finely graduated, the amount of mercury forced out of the vessel may be accurately measured.<Illustration on the right>.
This is the principle of the common mercurial thermometer, the construction of which will be afterwards more minutely described. At present we consider it simply as an instrument the indications of which vary when the temperature varies, but are always the same when the temperature of the instrument is the same.
The dilatation of other liquids, as well as that of solids and of gases, may be used for thermometric purposes, and the thermoelectric properties of metals, and the variation of their electric resistance with temperature 4) , are also employed in researches on heat. We must first, however, study the theory of temperature in itself before we examine the properties of different substances as related to temperature, and for this purpose we shall use the particular mercurial thermometer just described.
2 The mercurial thermometer. "Temperature" and "heat"
This thermometer consists of a glass tube terminating in a bulb, the bulb and part of the tube being filled with mercury, and the rest of the tube being empty. We shall suppose the tube to be graduated in any manner so that the height of the mercury in the tube may be observed and recorded. We shall not, however, assume either that the tube is of uniform section or that the degrees are of equal size, so that the scale of this primitive thermometer must be regarded as completely arbitrary 5) . By means of our thermometer we can ascertain whether one temperature is higher or lower than another, or equal to it, but we cannot assert that the difference between two temperatures, a and b, is greater or less than the difference between two other temperatures, c and d,).
We shall suppose that in every observation the temperature of the mercury and the glass is equal and uniform over the whole thermometer 6) . The reading of the scale will then depend on the temperature of the thermometer, and, since we have not yet established any more perfect thermometric scale, we shall call this reading provisionally 'the temperature by the arbitrary scale of the thermometer'.
The reading of a thermometer indicates primarily its own temperature, but if we bring the thermometer into intimate contact with another substance, as for instance if we plunge it into a liquid for a sufficient time, we find that the reading of the thermometer becomes higher or lower according as the liquid is hotter or colder than the thermometer, and that if we leave the thermometer in contact with the substance for a sufficient time the reading becomes stationary 7). |  < These are the instructions for “how to measure temperatures correctly”. Sensors must have permanently good thermal contact with the substance to be measured. Here, for example, for measuring the temperature in a reference block with a calibrated measuring device: each reference sensor is permanently in thermal contact with an aluminium block by means of heat-conducting paste. > Text in illustration: Sensor is placed on this and thermally embedded. The next aluminium plate is placed on top of this, the entire construction is finally placed inside an aluminium cube |
Let us now take a vessel of water which we shall suppose to be at the temperature of the air, so that if left to itself it would remain at the same temperature. Take another smaller vessel of thin sheet copper or tin plate, and fill it with water, oil, or any other liquid, and immerse it in the larger vessel of water for a certain time. Then, if by means of our thermometer we register the temperatures of the liquids in the two vessels before and after the immersion of the copper vessel, we find that if they are originally at the same temperature the temperature of both remains the same, but that if one is at a higher temperature than the other, that which has the higher temperature becomes colder and that which has the lower temperature becomes hotter, so that if they continue in contact for a sufficient time they arrive at last at the same temperature, after which no change of temperature takes place
| The loss of temperature by the hot body is not in general equal to the gain of temperature by the cold body, but it is manifest that the two simultaneous phenomena are due to one cause, and this cause may be described as the passage of Heat from the hot body to the cold one. As this is the first time we have used the word “Heat”, let us examine what we mean by it. | < Temperature: state of thermal excitation of a substance. Measured using a thermometer. remains the same even if I am observing only parts of the substance. Heat: Thermal content 9) , which the substance contains. Measured using a calorimeter (e.g. heat meter). doubles with twice the amount of substance. > |
We find the cooling of a hot body and the heating of a cold body happening simultaneously as parts of the same phenomenon, and we describe this phenomenon as the passage of heat from the hot body to the cold one. Heat, then, is something which may be transferred from one body to another, so as to diminish the quantity of heat in the first and increase that in the second by* the same amount. When heat is communicated to a body, the temperature of the body is generally increased, but sometimes other effects are produced, such as change of state 10) ; When heat leaves a body, there is either a fall of temperature or a change of state. If no heat enters or leaves a body, and if no changes of state or mechanical actions take place in the body, the temperature of the body will remain constant 11).
Heat, therefore, may pass out of one body into another just as water may be poured from one vessel into another, and it may be retained in a body for any time, just as water may be kept in a vessel. We have therefore a right to speak of heat as of a measurable quantity and to treat it mathematically like other measurable quantities so long as it continues to exist as heat. We shall find, however, that we have no right to treat heat as a substance for it may be transformed into something which is not heat, and is certainly not a substance at all, namely, mechanical work. We must remember, therefore, that though we admit heat to the title of a measurable quantity, we must not give it rank as a substance, but must hold our minds in suspense till we have further evidence as to the nature of heat.
'Heat' is a form of energy
Such evidence is furnished by experiments on friction, in which mechanical work, instead of being transmitted from one part of a machine to another, is apparently lost, while at the same time, and in the same place, heat is generated, the amount of heat being in an exact proportion to the amount of work lost 12) . We have, therefore, reason to believe that heat is of the same nature as mechanical work, that is, it is one of the forms of Energy.
<Illustration on the right: Racing cars build up a high amount of kinetic energy (typically mechanical). During braking this energy (the entire energy difference!) is converted into the energy form “heat” 13) . This picture by Nic Redhead shows the glowing brake disks of a racing car. J.P. Joule was the first to clearly describe the conversion of mechanical energy into heat and to experimentally determine the “heat equivalent” - this is what Maxwell draws on. Today, this phenomenon is “general knowledge”, even though it consequence is not really responsibly applied: it would be better to greatly reduce the huge downcycling of valuable kinetic energy into worthless heat that is ultimately discharged into the environment, which is possible e.g. through dynamic braking with an electric car if braking does not have to take place in necessary in an overly “sportive” manner. Used under CC BY-SA 2.0; part of original photograph.> >
<Quote: Maxwell: Heat CANNOT be a substance> 14) In the 18th century, when many new facts were being discovered relating to the action of heat on bodies, and when at the same time great progress was being made in the knowledge of the chemical action of substances increased at the same time, the word “Caloric” was introduced to signify heat as a measurable quantity. So long as the word denoted nothing more than this, it might be usefully employed, but the form of the word accommodated itself to the tendency of the chemists of that time to seek for new 'imponderable substances', so that the word caloric came to connote not merely heat, but heat as an indestructible imponderable fluid, insinuating itself into the pores of bodies, dilating and dissolving them, and ultimately vaporising them, combining with bodies in definite quantities, and so becoming latent, and reappearing when these bodies alter their condition. In fact, the word caloric, when once introduced, soon came to imply the recognised existence of something material, though probably of a more subtle nature than the then newly discovered gases. Caloric resembled these gases in being invisible and in its property of becoming fixed in solid bodies. It differed from them because its weight could not be detected by the finest balances, but there was no doubt in the minds of many eminent men that caloric was a fluid pervading all bodies, probably the cause of all repulsion and possibly even of the extension bodies in space. Since ideas of this kind have always been connected with the word caloric, and the word itself has been in no slight degree the means of embodying and propagating these ideas, and since all these ideas are now known to be false, we shall avoid as much as possible the use of the word caloric in treating of heat. We shall find it useful, however, when we wish to refer to the erroneous theory which supposes heat to be a substance, to call it the 'caloric theory of heat'. <End quote>
The word heat, though a primitive word and not a scientific term, will be found sufficiently free from ambiguity when we use it to express a measurable quantity, because it will be associated with words expressive of quantity, indicating how much heat we are speaking of
We have nothing to do with the word heat as an abstract term signifying the property of hot things, and when we might say a certain heat, as the heat of new milk, we shall always use the more scientific word temperature, and speak of the temperature of new milk.
We shall never use the word heat to denote the sensation of heat“ 15).
When we require an adjective to denote that a phenomenon is related to heat we shall call it a thermal phenomenon, as, for instance, we shall speak of the thermal conductivity of a substance or of thermal radiation to distinguish the conduction and radiation of heat from the conduction of electricity or the radiation of light” 16) . The science of heat has been called Thermotics, and the theory of heat as a form of energy is called Thermodynamics. In the same way the theory of the equilibrium of heat might be called Thermostatics, and that of the motion of heat Thermokinematics.
The instrument by which the temperature of bodies is registered is called a Thermometer or measurer of warmth, and the method of constructing and using thermometers may be called Thermometry.
The instrument by which quantities of heat are measured is called a Calorimeter , probably because it was invented at a time when heat was called Caloric. The name, however, is now well established, and is a convenient one, as its form is sufficiently distinct from that of the word Thermometer. The method of measuring heat may be called Calorimetry.
A certain quantity of heat, with which all other quantities are compared, is called a Thermal Unit . This is the quantity of heat required to produce a particular effect, such as to melt a pound of ice, or to raise a pound of water from one defined temperature to another defined temperature. A particular thermal unit has been called by some authors a Calorie. < Because we have firmly internalised the similarity of nature of heat and energy today, for some time we have been consistently using the unit of energy also for measuring heat quantities, that is the Joule J in the SI system (note: a traditional “calorie” 1 cal = 4.186 J) >
We have now obtained two of the fundamental ideas of the science of heat — the idea of temperature, or the property of a body considered with reference to its power of heating other bodies; and the idea of heat as a measurable quantity <proportional to quantity>, which may be transferred from hotter bodies to colder ones. We shall consider the further development of these ideas in the chapters on Thermometry and Calorimetry; but we must first direct our attention to the process by which heat is transferred from one body to another.
This process is called the Diffusion of Heat The diffusion of heat invariably transfers heat from a hotter body to a colder one23) , so as to cool the hotter body and warm the colder body. This process would go on till all bodies were brought to the same temperature if it were not for certain other processes by which the temperatures of bodies are changed inde- pendently of any exchange of heat with other bodies, as, for instance, when combustion or any other chemical process takes place, or when any change occurs in the form, structure, or physical state of the body.
The changes of temperature of a body arising from other causes than the transfer of heat from other bodies will be considered when we come to describe the different physical states of bodies. We are at present concerned only with the passage of heat into the body or out of it, and this always takes place by diffusion, and is always from a hotter to a colder body.
Three processes of diffusion of heat are commonly recognised - Conduction, Convection, and Radiation. In Radiation, the hotter body loses heat, and the colder body receives heat by means of a process occurring in some intervening medium which does not itself become thereby hot.
- Conduction
is the flow of heat through an unequally heated body from places of higher to places of lower temperature. - Convection
is the motion of the hot body itself carrying its heat with it. If by this motion it is brought near bodies colder than itself it will warm them faster than if it had not been moved nearer to them. The term convection is applied to those processes by which the diffusion of heat is rendered more rapid by the motion of the hot substance from one place to another, though the ultimate transfer of heat may still take place by conduction <in the special case where the substance in motion is a fluid we speak of convection>. - In Radiation
the hotter body loses heat, and the colder body receives heat {by means of a process occurring in some intervening medium which does not itself become thereby hot.} <electromagnetic waves> 17)
In each of these three processes of diffusion of heat the temperatures of the bodies between which the process takes place tend to become equal. We shall not at present discuss the convection of heat, because it is not a purely thermal phenomenon, since it depends on a hot substance being carried from one place to another, either by human effort, as when a hot iron is taken out of the fire and put into the tea-urn, or by some natural property of the heated substance, as when water, heated by contact with the bottom of a kettle placed on the fire, expands as it becomes warmed, and forms an ascending current, making way for colder and therefore denser water to descend and take its place. In every such case of convection the ultimate and only direct transfer of heat is due to conduction, and the only effect of the motion of the hot substance is to bring the unequally heated portions nearer to each other, so as to facilitate the exchange of heat.
<End of extract from Maxwell's “Theory of heat”>
Kinetic theory of heat
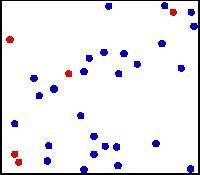
Thanks to Maxwell we now know “where” the energy goes when it is converted into thermal energy: it is particularly due to friction that kinetic energy is unevenly transferred to individual molecules of the relevant substance. These take kinetic as well as oscillation energy and pass them on to neighbouring molecules. This always happens in a random ('dissipative') form, in which the energy is “evenly” spread (more about this later) across all energy-receptive components. This description is called the “kinetic theory of heat”. Thermal energy is thus nothing other than the random kinetic / rotational / oscillation energy of matter. In qualitative terms we can illustrate this using the model gas experiment (on the right; click on the illustration to show the animation). A couple of hundred small steel balls are enough to let a uniform randomly moving jumble of balls appear. There are about 2⋅1021 air molecules (2 sextillion) in this visible volume!
This model of heat as a random motion of molecules explains in an easy way all of the properties and behaviour patterns of heat and temperature as elucidated above by Maxwell: if we understand temperature as the intensity of the heat motion (more exactly: the means of kinetic energy of each molecule) and heat as the total of all energies of the molecule in the observed system, then at once it intuitively becomes clear that:
- Heat (kinetic energy) is transferred when two systems come into contact; and it is the same amount that leaves one system and enters the other system (due to the law of energy conservation of mechanics, because the energy cannot go anywhere else).
- In a net exchange, heat transitions from systems with a higher temperature (rapid motion) into the system with a lower temperature.
- The net carry-over amount is zero if the temperatures are identical.
- With constant proportions of the mixture of substances the energy quantities are proportional to the number of molecules (that is, to the mass).
- Heat can be transferred through conduction (impact of movement from molecule to molecule,
- …entrainment (transfer of the more rapidly moving molecules to another place) and
- radiation (electromagnetic waves emitted from the electrically charged molecules that are moving around); and the net transfer is always in the direction towards the system with the lower temperatures (less intense movement).
The usefulness of this model goes much further than just these qualitative statements, however. With a little bit of classic physics and a few calculation transformations, it is possible to make quantitative predictions with a high accuracy regarding e.g. the processes in gases18) from this model; effects that initially seem mysterious immediately become understandable 19) . We will deal with this more closely further on, again following Maxwell - for all those who have always wanted to see the mysteries of thermodynamics revealed.
Next section "Heat transfer coefficient" 🌡️
Back to building physics basic course on "Heat" - Overview 🌡️ (only in German)
Literature
THEORY OF HEAT BY J. C. MAXWELL, CAMBRIDGE UNIVERSITY PROFESSOR, document is publicly accessible, e.g. under the name Theory of Heat and is interesting not only for historic reasons.